Can you Copperplate?
This lesson explores chemical engineering and how the processes of chemical plating and electroplating have impacted many industries. Students work in teams to copper plate a range of items using everyday materials.
- Learn about engineering design and redesign.
- Learn about chemical engineering.
- Learn how engineering can help solve society’s challenges.
- Learn about teamwork and problem solving.
Age Levels: 12-18
Safety Note:
Have students wear rubber gloves when removing the materials from the solution or when disposing of the solution. This activity should be done in a well-ventilated area as the solution can give off an odor after the plating process.
- Do not use valuable coins, as the finish will be altered and may impact collectible coin value.
- Students should not drink the lemon juice or vinegar solution either before or after coins have been submerged.
Build Materials (For each team)
Required Materials (per team)
- Rubber gloves
- Glass jars (jelly or canning jars work well)
- 25 pennies, euros, or any other coin with copper coating
Required Materials (Table of Possibilities)
- Iron nails, screws or bolts (not galvanized)
- Salt
- White/clear vinegar
- Lemon juice
- Orange juice
- Baking soda
- Scouring pad
- Water
Testing Materials
- Rubber gloves
- Water source
- Bucket or sink area
- Hundreds of dirty pennies, euros or other coins or materials with a high copper surface content
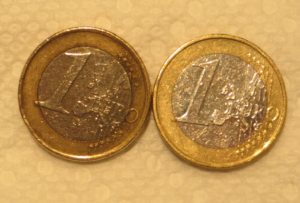
Materials
- Rubber gloves
- Water source
- Bucket or sink area
- Hundreds of dirty pennies, euros or other coins or materials with a high copper surface content

Process
Several methods will be successful at removing a layer of copper from the pennies or other copper surfaced items and transferring the copper to another metal item. See a suggested solution below. Consider if you want to provide these methods to the teams or whether you would like teams to come up with a solution on their own. Be sure to review and supervise ALL solutions developed for safety.
- Solution:
- Put 25 pennies (or other coins or materials with high external copper content) into a glass jar with 1/2 cup (125 ml) of white vinegar and 1/4 teaspoon (1ml) of salt.
- Let the solution sit for 5 minutes, then add an iron nail, screw, or other item (not galvanized) for another 5 minutes.
- Remove the iron item and use a scouring pad to remove some of the coating and then place back in the jar.
- Add the coins and let the solution sit for 15 minutes.
- The coins should be shiny and the iron item should have a thin coating of copper.
Dispose of solutions in a sink (they will likely have a strong smell). If the pennies are not rinsed in water after the testing, they will turn bluegreen after a few days.
Design Challenge
You are part of a team of engineers given the challenge of applying a copper surface to another metal. You can choose the metal item(s) you want to plate and also the chemical solution and timing you think will work best. You will develop two different methods and see which works the best.
Criteria
- Must develop two different copper plating methods
- Must use a glass container
Constraints
- Use only the materials provided
- Break class into teams of 2-4.
- Hand out the Can You Copperplate worksheet, as well as some sheets of paper for sketching designs.
- Discuss the topics in the Background Concepts Section. Consider showing students several different screws or nails with different finishes and ask them why different finishes are manufactured.
- Review the Engineering Design Process, Design Challenge, Criteria, Constraints and Materials.
- Provide each team with their materials.
- Explain that students must develop two different plans for plating one of their items. They may use different solutions, materials, timings, etc. Each must use older pennies, euros, or other coins or materials with high external copper content – and use a glass container.
- Step 1: Identify the items and determine two methods for copper plating those items. You may want to consider placing multiple items in one of the solutions. But you should be aware that any copper released from the pennies or other copper based coins, will then be diluted between multiple metal items. Document the following for each of the 2 solutions:
- Describe solution (include the quantity of each item that will be included
- Describe items to be plated
- Describe method (timing, rinsing methods, etc.)
- Anticipated result
- Step 2: Present your plan and anticipated outcome to the class. Consider the ideas of other teams and adjust your plan, if you want.
- Step 3: Test the two methods and note your observations for each:
- What happened to the solution?
- What happened to the item(s) you were trying to plate?
- What happened to the pennies or copper surfaced items/coins?
- Did this method work?
- Announce the amount of time they have to complete Step 1 (1 hour recommended).
- Use a timer or an on-line stopwatch (count down feature) to ensure you keep on time. (www.online-stopwatch.com/full-screen-stopwatch). Give students regular “time checks” so they stay on task. If they are struggling, ask questions that will lead them to a solution quicker.
- Students meet and develop a plan. They agree on materials and methods.
- Teams complete Step 1 planning.
- Teams complete Step 2 presentation.
- Teams complete Step 3 testing and observations. See “Testing Materials and Process” section.
- Why does this process work?
- The mixture of vinegar and salt creates an acid solution. Copper oxide dissolves when exposed to the solution.
- The iron materials are coated with copper because when the pennies are in the solution some of the surface copper will dissolve. But when copper atoms leave the penny, they leave some of their electrons behind….really positively charged copper ions (copper atoms that are missing two electrons).
- Some of the metal in the iron dissolves and there are positively charged iron ions floating in the solution with the positively charged copper ions. When the iron ions leave the nail/screw, the item becomes negatively charged and so attracts the positively charged ions in the solution.
- Copper ions are more strongly attracted to the iron item than the iron atoms, so they coat the item with a thin coating of copper.
- As a class, discuss the student reflection questions.
- For more content on the topic, see the “Digging Deeper” section.
Student Reflection (engineering notebook)
- Was your team able to copperplate a metal item? What factors do you think contributed to the success or failure of your method?
- If you found you needed to make changes to your method after listening to the methods planned by other teams, describe why your team decided to make revisions.
- Which method that another team adopted was the most successful? Why do you think this method worked so well?
- Do you think that this activity was more rewarding to do as a team, or would you have preferred to work alone on it? Why?
- Do you think that chemical engineers have to make many attempts to achieve a goal? What do you think it would be like to fail over and over before having success?
- What industry or business do you think might want to use the method you developed?
Time Modification
The lesson can be done in as little as 1 class period for older students. However, to help students from feeling rushed and to ensure student success (especially for younger students), split the lesson into two periods giving students more time to brainstorm, test ideas and finalize their design. Conduct the testing and debrief in the next class period.
Plating Processes

Metal Plating
Plating of metals has been done via many processes since ancient times. Gold plating is a method of depositing a thin layer of gold onto the surface of another metal, most often copper or silver (to make silver-gilt), by chemical or electrochemical plating. Electroplating has many uses….it can make a material more resistant to damage or abrasion….it can make a material look nicer….to harden a surface…to reduce friction….to improve paint adhesion, to alter conductivity, for radiation shielding, and for other purposes. It is also used to build up the thickness of undersized parts. It is widely used in industry for coating metal objects with a thin layer of a different metal. The layer of metal deposited has some desired property, which the metal of the object lacks. For example, chromium plating is done on many objects such as car parts, bath taps, kitchen gas burners, wheel rims and many others. Gold is often plated onto silver or a less expensive metal to reduce the cost of jewelry. Gold plating is often used in electronics, to provide a corrosion-resistant electrically conductive layer on copper, typically in electrical connectors and printed circuit boards. The surface of nails used in construction are sometimes galvanized which adds a layer of zinc.
What is Electroplating?
Electroplating is a process in which metal ions in a solution are moved by an electric field to coat an electrode. The process uses electrical current to coat a conductive object with a thin layer of the material, such as a metal. The process used in electroplating is called electrodeposition. In one technique, the anode is made of the metal to be plated on the part. Both components are immersed in a solution called an electrolyte containing one or more dissolved metal salts as well as other ions that permit the flow of electricity. A power supply supplies a direct current to the anode, oxidizing the metal atoms that comprise it and allowing them to dissolve in the solution.
At the cathode, the dissolved metal ions in the electrolyte solution are reduced at the interface between the solution and the cathode, such that they “plate out” onto the cathode. The rate at which the anode is dissolved is equal to the rate at which the cathode is plated, vis-a-vis the current flowing through the circuit. In this manner, the ions in the electrolyte bath are continuously replenished by the anode. In the example to the right, in an acid solution, copper is oxidized at the anode to Cu2+ by losing two electrons. The Cu2+ associates with the anion SO42- in the solution to form copper sulfate.
- Chemical: Any substance (as an acid) that is formed when two or more other substances act upon one another or that is used to produce a change in another substance.
- Constraints: Limitations with material, time, size of team, etc.
- Copper: A reddish brown metal that is one of the chemical elements
- Copperplate: Applying a copper surface to another metal.
- Criteria: Conditions that the design must satisfy like its overall size, etc.
- Engineers: Inventors and problem-solvers of the world. Twenty-five major specialties are recognized in engineering (see infographic).
- Engineering Design Process: Process engineers use to solve problems.
- Engineering Habits of Mind (EHM): Six unique ways that engineers think.
- Iteration: Test & redesign is one iteration. Repeat (multiple iterations).
- Prototype: A working model of the solution to be tested.
- Solution: A mixture of two or more substances that stays evenly mixed.
[/vc_column_text]
Internet Connections
Recommended Reading
- Electroplating Engineering Handbook (ISBN: 978- 1475708561)
- The Polishing and Plating of Metals (ISBN: 978- 1246867176)
- Electro-deposition of Metals (ISBN: 978- 1176590250)
Writing Activity
Write an essay or a paragraph about why nails used in construction are galvanized.
Alignment to Curriculum Frameworks
Note: Lesson plans in this series are aligned to one or more of the following sets of standards:
- U.S. Science Education Standards (http://www.nap.edu/catalog.php?record_id=4962)
- U.S. Next Generation Science Standards (http://www.nextgenscience.org/)
- International Technology Education Association’s Standards for Technological Literacy (http://www.iteea.org/TAA/PDFs/xstnd.pdf)
- U.S. National Council of Teachers of Mathematics’ Principles and Standards for School Mathematics (http://www.nctm.org/standards/content.aspx?id=16909)
- U.S. Common Core State Standards for Mathematics (http://www.corestandards.org/Math)
- Computer Science Teachers Association K-12 Computer Science Standards (http://csta.acm.org/Curriculum/sub/K12Standards.html)
National Science Education Standards Grades K-4 (ages 4-9)
CONTENT STANDARD A: Science as Inquiry
As a result of activities, all students should develop
- Abilities necessary to do scientific inquiry
- Understanding about scientific inquiry
CONTENT STANDARD B: Physical Science
As a result of the activities, all students should develop an understanding of
- Properties of objects and materials
CONTENT STANDARD D: Earth and Space Science
As a result of their activities, all students should develop an understanding of
- Properties of earth materials
CONTENT STANDARD E: Science and Technology
As a result of activities, all students should develop
- Abilities of technological design
- Understanding about science and technology
CONTENT STANDARD F: Science in Personal and Social Perspectives
As a result of activities, all students should develop understanding of
- Types of resources
- Science and technology in local challenges
CONTENT STANDARD G: History and Nature of Science
As a result of activities, all students should develop understanding of
- Science as a human endeavor
National Science Education Standards Grades 5-8 (ages 10-14)
CONTENT STANDARD A: Science as Inquiry
As a result of activities, all students should develop
- Abilities necessary to do scientific inquiry
CONTENT STANDARD B: Physical Science
As a result of their activities, all students should develop an understanding of
- Properties and changes of properties in matter
CONTENT STANDARD E: Science and Technology
As a result of activities in grades 5-8, all students should develop
- Abilities of technological design
CONTENT STANDARD F: Science in Personal and Social Perspectives
As a result of activities, all students should develop understanding of
- Science and technology in society
National Science Education Standards Grades 5-8 (ages 10-14)
CONTENT STANDARD G: History and Nature of Science
As a result of activities, all students should develop understanding of
- Science as a human endeavor
- History of science
National Science Education Standards Grades 9-12 (ages 14-18)
CONTENT STANDARD A: Science as Inquiry
As a result of activities, all students should develop
- Abilities necessary to do scientific inquiry
CONTENT STANDARD B: Physical Science
As a result of their activities, all students should develop understanding of
- Chemical Reactions
- Structure and properties of matter
CONTENT STANDARD E: Science and Technology
As a result of activities, all students should develop
- Abilities of technological design
- Understandings about science and technology
CONTENT STANDARD F: Science in Personal and Social Perspectives
As a result of activities, all students should develop understanding of
- Natural resources
- Science and technology in local, national, and global challenges
CONTENT STANDARD G: History and Nature of Science
As a result of activities, all students should develop understanding of
- Science as a human endeavor
- Historical perspectives
Next Generation Science Standards – Grades 2-5 (Ages 7-11)
Matter and its Interactions
- 2-PS1-2. Analyze data obtained from testing different materials to determine which materials have the properties that are best suited for an intended purpose.
Engineering Design
Students who demonstrate understanding can:
- 3-5-ETS1-1.Define a simple design problem reflecting a need or a want that includes specified criteria for success and constraints on materials, time, or cost.
- 3-5-ETS1-2.Generate and compare multiple possible solutions to a problem based on how well each is likely to meet the criteria and constraints of the problem.
- 3-5-ETS1-3.Plan and carry out fair tests in which variables are controlled and failure points are considered to identify aspects of a model or prototype that can be improved.
Next Generation Science Standards – Grades 6-8 (Ages 11-14)
Matter and its Interactions
- MS-PS1-2. Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred.
Engineering Design
Students who demonstrate understanding can:
- MS-ETS1-1. Define the criteria and constraints of a design problem with sufficient precision to ensure a successful solution, taking into account relevant scientific principles and potential impacts on people and the natural environment that may limit possible solutions.
- MS-ETS1-2. Evaluate competing design solutions using a systematic process to determine how well they meet the criteria and constraints of the problem.
Standards for Technological Literacy – All Ages
The Nature of Technology
- Standard 1: Students will develop an understanding of the characteristics and scope of technology.
- Standard 3: Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
Technology and Society
- Standard 4: Students will develop an understanding of the cultural, social, economic, and political effects of technology.
- Standard 6: Students will develop an understanding of the role of society in the development and use of technology.
- Standard 7: Students will develop an understanding of the influence of technology on history.
Design
- Standard 8: Students will develop an understanding of the attributes of design.
- Standard 9: Students will develop an understanding of engineering design.
- Standard 10: Students will develop an understanding of the role of troubleshooting, research and development, invention and innovation, and experimentation in problem solving.
Abilities for a Technological World
- Standard 11: Students will develop abilities to apply the design process.
- Standard 13: Students will develop abilities to assess the impact of products and systems.

Engineering Teamwork and Planning
You are part of a team of engineers given the challenge of applying a copper surface to another metal. You can choose the metal item/items you want to plate and also the chemical solution and timing you think will work best. You will develop two different approaches and see which works best.
Step 1: Determine two methods for copper plating and identify your items. Note, you may wish to consider placing multiple items in one of the solutions…but be aware that any copper released from the pennies or other copper based coins you have will then be diluted between multiple metal items and so you may not see results. In the box below, describe your solution, method, timing, and the items you will copper plate.
| Solution | Describe Solution (include quantity of each item you will include) | Describe items to be plated | Describe method (including timing, rinsing methods, etc.) | Anticipated Result |
| 1 |
|
|||
| 2 |
|

Presentation and Testing
Step 2: Present your plan and anticipated outcome to the class. Consider the ideas of the other teams and adjust your plan if you like.
Step 3: Test your two methods and note your observations in the box below.
| Solution | What Happened to the Solution? | What Happened to the Item you were trying to plate? | What happened to the pennies or copper surfaced items/coins? | Did this method work? |
| 1 |
|
|||
| 2 |
|

Reflection
Complete the reflection questions below:
- Was your team able to copperplate a metal item? What factors do you think contributed to the success or failure of your method?
- If you found you needed to make changes to your method after listening to the methods planned by other teams, describe why your team decided to make revisions.
- Which method that another team adopted was the most successful? Why do you think this method worked so well?
- Do you think that this activity was more rewarding to do as a team, or would you have preferred to work alone on it? Why?
- Do you think that chemical engineers have to make many attempts to achieve a goal? What do you think it would be like to fail over and over before having success?
- What industry or business do you think might want to use the method you developed?




 Industrial Engineering
Industrial Engineering
 Computer Engineering
Computer Engineering